Chemistry, 09.07.2020 01:01 shifaxoxoxo
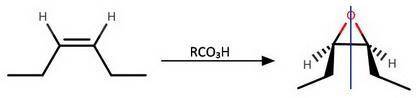
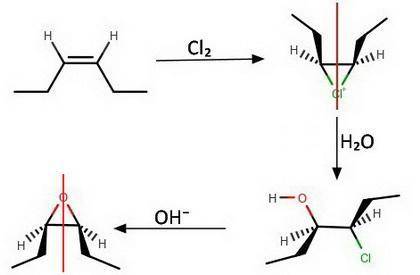
The two reactions above, show routes for conversion of an alkene into an oxirane. If the starting alkene is cis-3-hexene the configurations of the oxirane products, A and B are Product A: Product B: Will either of these two oxirane products rotate the plane of polarization of plane polarized light?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
The two reactions above, show routes for conversion of an alkene into an oxirane. If the starting al...
Questions

Mathematics, 05.05.2021 23:40

Mathematics, 05.05.2021 23:40

Physics, 05.05.2021 23:40

Computers and Technology, 05.05.2021 23:40

Mathematics, 05.05.2021 23:40


English, 05.05.2021 23:40

Biology, 05.05.2021 23:40

History, 05.05.2021 23:40





Mathematics, 05.05.2021 23:40


Mathematics, 05.05.2021 23:40

Mathematics, 05.05.2021 23:40



Mathematics, 05.05.2021 23:40