Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1


Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
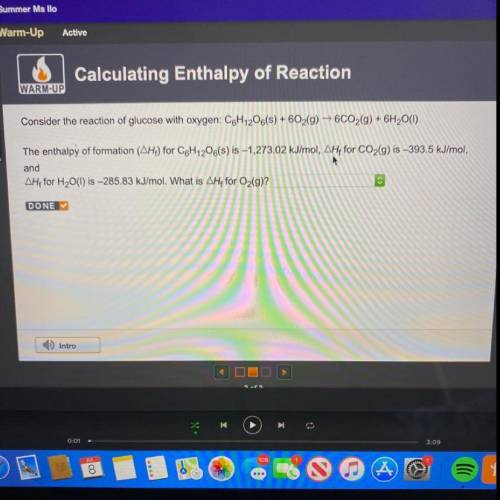
Consider the reaction of glucose with oxygen: C6H12O6(s) + 602(g) → 6CO2(g) + 6H2O(1)
The enthalpy...
Questions


Mathematics, 14.04.2020 04:30



Mathematics, 14.04.2020 04:30

Chemistry, 14.04.2020 04:30

History, 14.04.2020 04:30


Computers and Technology, 14.04.2020 04:30



English, 14.04.2020 04:30

Mathematics, 14.04.2020 04:30

Mathematics, 14.04.2020 04:30


English, 14.04.2020 04:30