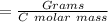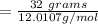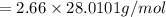Chemistry, 13.07.2020 17:01 hazeleyes2006
Fe2O3(s)+3C(s)→2Fe(s)+3CO(g) how many grams of CO are produced when 32.0 grams of C react

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Fe2O3(s)+3C(s)→2Fe(s)+3CO(g) how many grams of CO are produced when 32.0 grams of C react...
Questions


English, 06.03.2021 01:00


Mathematics, 06.03.2021 01:00



Mathematics, 06.03.2021 01:00


Mathematics, 06.03.2021 01:00

Mathematics, 06.03.2021 01:00


Social Studies, 06.03.2021 01:00



Mathematics, 06.03.2021 01:00


English, 06.03.2021 01:00



Mathematics, 06.03.2021 01:00