
Chemistry, 14.07.2020 19:01 samariahmiddlebrooks
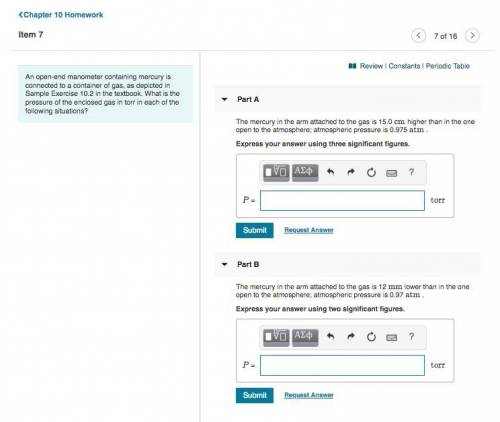
An open-end manometer containing mercury is connected to a container of gas, as depicted in Sample Exercise 10.2 in the textbook. What is the pressure of the enclosed gas in torr in each of the following situations? Part A: The mercury in the arm attached to the gas is 15.0 cm higher than in the one open to the atmosphere; atmospheric pressure is 0.975 atm . Part B: The mercury in the arm attached to the gas is 12 mm lower than in the one open to the atmosphere; atmospheric pressure is 0.97 atm.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2
You know the right answer?
An open-end manometer containing mercury is connected to a container of gas, as depicted in Sample E...
Questions



Biology, 25.02.2021 18:30


Chemistry, 25.02.2021 18:30

Mathematics, 25.02.2021 18:30

English, 25.02.2021 18:30



History, 25.02.2021 18:30

Spanish, 25.02.2021 18:30


Mathematics, 25.02.2021 18:30




Biology, 25.02.2021 18:30

Social Studies, 25.02.2021 18:30


Mathematics, 25.02.2021 18:30



