
Chemistry, 13.07.2020 20:01 WolfMeadows
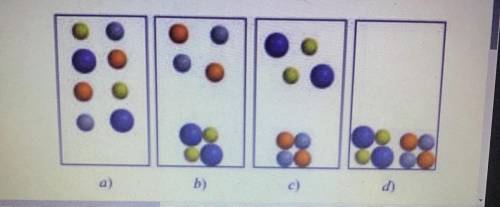
PLEASE! I HAVE 20 MINS LEFT :( Two aqueous solutions of AgNO3 and NaCl are mixed. Which of the following diagrams best represents the mixture? For simplicity, water molecules are not shown (Ag + = gray, Cl- = orange, Na + = green, NO ^ - 3 = blue) PLEASE I NEED HELP I ONLY HAVE 15 MINS PLS :'((

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 15:30
Amole, which is a unit in chemistry, contains 6.02 x 1023 atoms or particles. how many zeroes follow the 2 when this number is written out in standard form? a) 21 b) 23 c) 24 d) 25
Answers: 1
You know the right answer?
PLEASE! I HAVE 20 MINS LEFT :( Two aqueous solutions of AgNO3 and NaCl are mixed. Which of the follo...
Questions


Social Studies, 31.01.2020 07:53




Chemistry, 31.01.2020 07:53




Mathematics, 31.01.2020 07:53


Mathematics, 31.01.2020 07:53

Biology, 31.01.2020 07:53


Advanced Placement (AP), 31.01.2020 07:53

Mathematics, 31.01.2020 07:53


Mathematics, 31.01.2020 07:53

Mathematics, 31.01.2020 07:53

History, 31.01.2020 07:53



