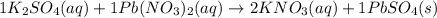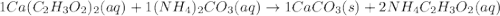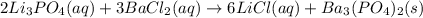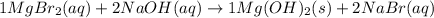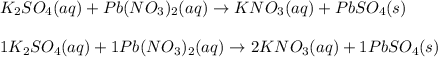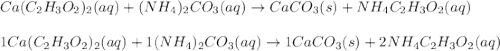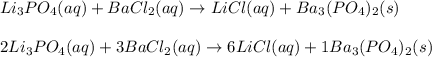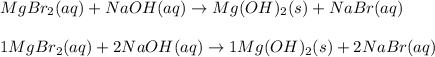Chemistry, 14.07.2020 18:01 babyduckies37
Balance the given molecular equations in aqueous solution with coefficients and phases for each species. If you determine that a species should have a coefficient of 1, you must indicate this with the "1" label. Do not leave the coefficient space blank for any species in the balanced equation.
1 2 3 4 5 6 s aq
K2SO4 ( )+ Pb(NO3)2 ( ) → KNO3 ( )+ PbSO4 ( )
Ca(C2H3O2)2 ( )+ (NH4)2CO3 ( ) → CaCO3()+ NH4C2H3O2()
Li3PO4( )+ BaCl2 ( ) → LiCl ( ) + Ba3(PO4)2 ( )
MgBr2( ) + NaOH ( ) → Mg(OH)2 ( ) + NaBr ( )

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Balance the given molecular equations in aqueous solution with coefficients and phases for each spec...
Questions




Business, 23.01.2020 00:31

Mathematics, 23.01.2020 00:31









Physics, 23.01.2020 00:31


Geography, 23.01.2020 00:31