

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
You know the right answer?
Question 3 (11 points)
A gas has a volume of 690.0mL at -15.1°C and 392.0 mmHg. What would the volu...
Questions




Mathematics, 11.10.2019 10:20

Mathematics, 11.10.2019 10:20

History, 11.10.2019 10:20


English, 11.10.2019 10:20



Mathematics, 11.10.2019 10:20



Mathematics, 11.10.2019 10:20





Mathematics, 11.10.2019 10:20

Computers and Technology, 11.10.2019 10:20

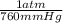 = 0.516 atm
= 0.516 atm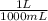 = 0.69 L
= 0.69 L

