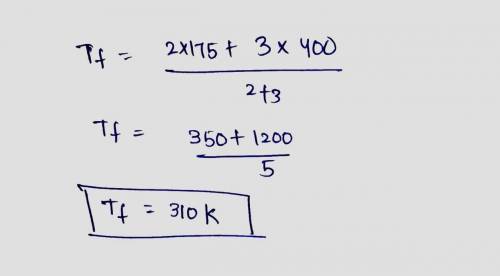Chemistry, 15.07.2020 01:01 queenb1416
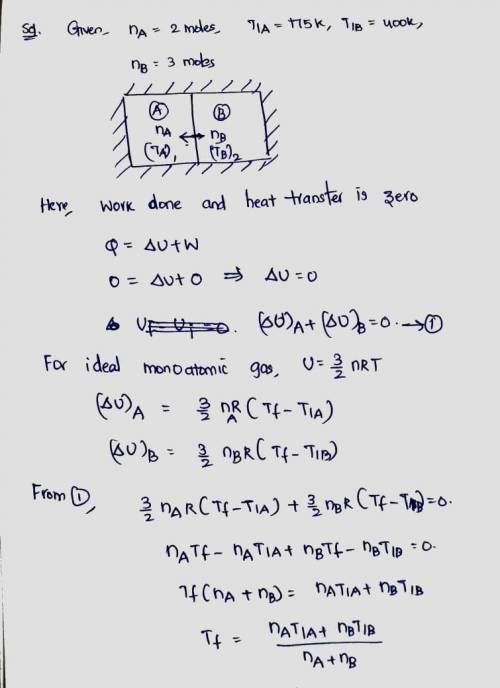
Two systems of monotonic ideal gas are separated by a diathermal wall. In system A there are 2 moles initially at 175 K, in system B there are 3 moles initially at 400 K. Write U_AU A and U_BU B as functions of RR and the determine the common temperature (in Kelvin) after equilibrium is reached.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Two systems of monotonic ideal gas are separated by a diathermal wall. In system A there are 2 moles...
Questions

Mathematics, 25.09.2019 13:10

SAT, 25.09.2019 13:10


Mathematics, 25.09.2019 13:10

Mathematics, 25.09.2019 13:10




Biology, 25.09.2019 13:10

Mathematics, 25.09.2019 13:10




English, 25.09.2019 13:10

History, 25.09.2019 13:10



French, 25.09.2019 13:10


History, 25.09.2019 13:10