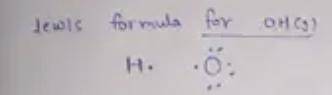Chemistry, 15.07.2020 01:01 joshuaburton773
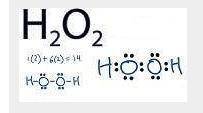
Many free radicals combine to form molecules that do not contain any unpaired electrons. The driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. Consider the formation of hydrogen peroxide. 2OH(g)⟶H2O2(g) Write Lewis formulas for the reactant and product species in the chemical equation. Include nonbonding electrons.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

You know the right answer?
Many free radicals combine to form molecules that do not contain any unpaired electrons. The driving...
Questions

Biology, 18.10.2019 23:30

Mathematics, 18.10.2019 23:30

Mathematics, 18.10.2019 23:30

History, 18.10.2019 23:30

Spanish, 18.10.2019 23:30


Mathematics, 18.10.2019 23:30


Mathematics, 18.10.2019 23:30


Spanish, 18.10.2019 23:30


History, 18.10.2019 23:30

Mathematics, 18.10.2019 23:30

Mathematics, 18.10.2019 23:30

World Languages, 18.10.2019 23:30

History, 18.10.2019 23:30

History, 18.10.2019 23:30