
Chemistry, 14.07.2020 01:01 robertotugalanp1wlgs
Write the chemical equation for the reaction whose enthalpy change is the standard enthalpy of formation of fructose, C6H12O6(s), ΔH∘f[C6H12O6].

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Write the chemical equation for the reaction whose enthalpy change is the standard enthalpy of forma...
Questions



Mathematics, 08.09.2020 03:01





Biology, 08.09.2020 03:01

English, 08.09.2020 03:01





Biology, 08.09.2020 03:01

Mathematics, 08.09.2020 03:01

Mathematics, 08.09.2020 03:01

Mathematics, 08.09.2020 03:01



Mathematics, 08.09.2020 03:01

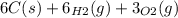
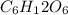
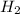 (s)
(s)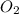 (s)
(s)

