Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Please help me fast
When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen r...
Questions


Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Business, 20.11.2020 19:50

Geography, 20.11.2020 19:50

World Languages, 20.11.2020 19:50

World Languages, 20.11.2020 19:50

Biology, 20.11.2020 19:50

Arts, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50



Business, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50


Biology, 20.11.2020 19:50

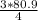 = 60.675
= 60.675