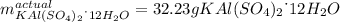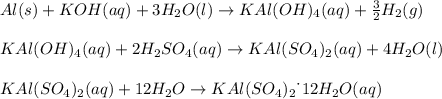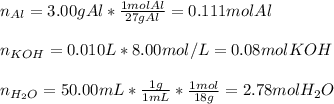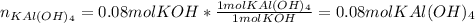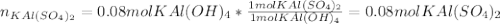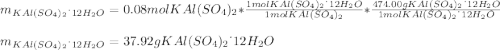Chemistry, 14.07.2020 23:01 sarahmkey6
given the following quantities of reactants and the balance equations; assuming an 85.0% yield, determine how many grams of potassium aluminum sulphate dodecahydrate can be produced.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
given the following quantities of reactants and the balance equations; assuming an 85.0% yield, dete...
Questions


Mathematics, 23.09.2020 05:01

Social Studies, 23.09.2020 05:01

English, 23.09.2020 05:01


History, 23.09.2020 05:01

Computers and Technology, 23.09.2020 05:01



Business, 23.09.2020 05:01


Physics, 23.09.2020 05:01



History, 23.09.2020 05:01

Social Studies, 23.09.2020 05:01

Engineering, 23.09.2020 05:01



Mathematics, 23.09.2020 05:01