
Chemistry, 14.07.2020 01:01 Prettygirlyaya
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40 at 346 K. (a) A pure sample of gaseous carbonyl bromide, COBr2(g), is introduced into a rigid flask at a temperature of 346 K so that its original pressure is 0.123 atm. Calculate the fraction of this starting material that is converted to products at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40...
Questions

Mathematics, 21.07.2020 08:01

Engineering, 21.07.2020 08:01


English, 21.07.2020 08:01

Mathematics, 21.07.2020 08:01





Mathematics, 21.07.2020 08:01


Mathematics, 21.07.2020 08:01






English, 21.07.2020 08:01


Chemistry, 21.07.2020 08:01

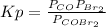 = 5.40
= 5.40

