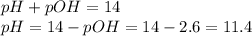Chemistry, 15.07.2020 01:01 29delphina
What is the pH of a 0.300 M NH₃ solution that has Kb = 1.8 × 10⁻⁵ ? The equation for the dissociation of NH₃ is: NH₃ (aq) + H₂O (l) ⇄ NH₄⁺ (aq) + OH⁻ (aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1


Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
What is the pH of a 0.300 M NH₃ solution that has Kb = 1.8 × 10⁻⁵ ? The equation for the dissociatio...
Questions

Mathematics, 23.05.2020 22:00




History, 23.05.2020 22:00


Mathematics, 23.05.2020 22:00

Mathematics, 23.05.2020 22:00










Mathematics, 23.05.2020 22:00


Mathematics, 23.05.2020 22:00

![[OH^{-} ]=\sqrt{Kb \times Cb } = \sqrt{1.8 \times 10^{-5} \times 0.300 } = 2.3 \times 10^{-3} M](/tpl/images/0706/3859/9462d.png)
![pOH =-log[OH^{-} ]= -log(2.3 \times 10^{-3} M) = 2.6](/tpl/images/0706/3859/e37b1.png)