

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
A neutralization experiment using NaOH and HCl produced 716J. The limiting reactant was NaOH, which...
Questions

Mathematics, 26.10.2020 17:00


Computers and Technology, 26.10.2020 17:00


Biology, 26.10.2020 17:00


Mathematics, 26.10.2020 17:00

Physics, 26.10.2020 17:00

Social Studies, 26.10.2020 17:00










Mathematics, 26.10.2020 17:00


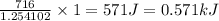 (1kJ=1000J)
(1kJ=1000J)


