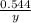Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

You know the right answer?
Small quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO3(s)...
Questions





Biology, 22.06.2019 17:00

Physics, 22.06.2019 17:00






Mathematics, 22.06.2019 17:00



Computers and Technology, 22.06.2019 17:00




Health, 22.06.2019 17:00

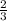 =
=