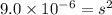Chemistry, 15.07.2020 02:01 heaven8606
The Ksp of calcium sulfate, CaSO4, is 9.0 × 10-6. What is the concentration of CaSO4 in a saturated solution? A. 3.0 × 10-3 Molar B. 9.0 × 10-3 Molar C. 3.0 × 10-6 Molar D. 9.0 × 10-6 Molar

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3
You know the right answer?
The Ksp of calcium sulfate, CaSO4, is 9.0 × 10-6. What is the concentration of CaSO4 in a saturated...
Questions

Mathematics, 11.06.2020 00:57







Mathematics, 11.06.2020 00:57

Physics, 11.06.2020 00:57



History, 11.06.2020 00:57







Health, 11.06.2020 00:57

English, 11.06.2020 00:57

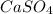 in a saturated solution is
in a saturated solution is 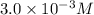
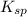
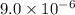
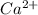 and 1 mole of
and 1 mole of 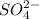
![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0706/6780/958f2.png)
![9.0\times 10^{-6}=[s][s]](/tpl/images/0706/6780/ee654.png)