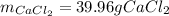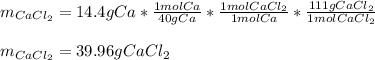Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2


Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
For the reaction Ca(s)+Cl2(g)→CaCl2(s) calculate how many grams of the product form when 14.4 g of C...
Questions




Biology, 07.05.2020 01:13


Mathematics, 07.05.2020 01:13











Mathematics, 07.05.2020 01:13