
Chemistry, 15.07.2020 01:01 Nathaliasmiles
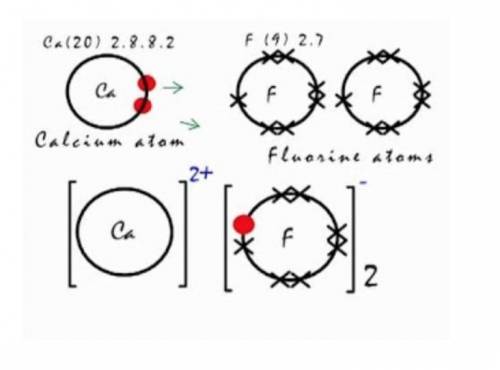
Q10. Calcium fluoride can be made from the reaction of calcium metal with fluorine gas. The image shows this reaction. Explain how the product is formed in terms of electron movement and what the final electron configurations are

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Q10. Calcium fluoride can be made from the reaction of calcium metal with fluorine gas. The image sh...
Questions

Social Studies, 20.02.2021 04:50

Mathematics, 20.02.2021 04:50



Mathematics, 20.02.2021 04:50


Mathematics, 20.02.2021 04:50






Mathematics, 20.02.2021 04:50

Mathematics, 20.02.2021 04:50

History, 20.02.2021 04:50

Chemistry, 20.02.2021 04:50


Mathematics, 20.02.2021 04:50


Mathematics, 20.02.2021 04:50



