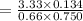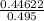When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of equilibrium concentrations of reactants and products) for the reaction below is 0.66 at 979°C. If the following concentrations are measured after the reaction reaches equilibrium, what is the concentration of CO(g) in the equilibrated mixture? answer will be in M
Component: Measured Equilibrium Concentration
A. H2 0 (g) 0.750 M
B. CO2 (g) 0.134 M
C. H2 (g) 3.33 M

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of e...
Questions

English, 10.07.2019 21:30

Mathematics, 10.07.2019 21:30




History, 10.07.2019 21:30

History, 10.07.2019 21:30

Social Studies, 10.07.2019 21:30

Mathematics, 10.07.2019 21:30


Mathematics, 10.07.2019 21:30

Mathematics, 10.07.2019 21:30


History, 10.07.2019 21:30


History, 10.07.2019 21:30

History, 10.07.2019 21:30



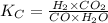
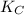 = equilibrium constant
= equilibrium constant