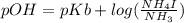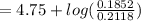Chemistry, 15.07.2020 02:01 kevinvalencia01
An aqueous solution contains 0.397 M ammonia. Calculate the pH of the solution after the addition of 4.63 x 10-2 moles of perchloric acid (HClO4) to 250 mL of this solution. (Assume the volume does not change upon adding perchloric acid). Ka = 5.7 x 10-10, Kb = 1.80 x 10-5

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
An aqueous solution contains 0.397 M ammonia. Calculate the pH of the solution after the addition of...
Questions

History, 27.09.2020 07:01


History, 27.09.2020 07:01



Mathematics, 27.09.2020 07:01








Biology, 27.09.2020 07:01


Mathematics, 27.09.2020 07:01

Mathematics, 27.09.2020 07:01



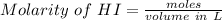
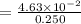
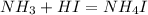
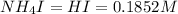
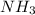 left is
left is