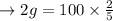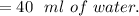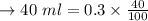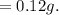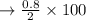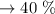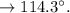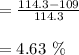Chemistry, 15.07.2020 02:01 enriquerer12
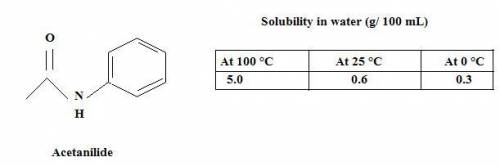
You need to purify 2.0 grams of an impure sample of Acetanilide. The sample is contaminated with aniline. After the purification is complete you isolate 0.8 grams of acetanilide and record a melting point range of 108-110 °C. Complete the following calculations and show your work.
a. Calculate the minimum amount of distilled water you would use to complete the recrystallization.
b. How much acetanilide will still be dissolved in solution even after the sample is cooled to 0 °C?
c. Calculate the % recovery and the % error for the melting point.
d. Why is the percent recovery less than 100%? Describe multiple sources for loss of sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
You need to purify 2.0 grams of an impure sample of Acetanilide. The sample is contaminated with ani...
Questions








Mathematics, 22.08.2020 23:01


Mathematics, 22.08.2020 23:01

History, 22.08.2020 23:01


Mathematics, 22.08.2020 23:01



Computers and Technology, 22.08.2020 23:01

Mathematics, 22.08.2020 23:01


History, 22.08.2020 23:01