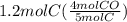Chemistry, 14.07.2020 03:01 xXCoryxKenshinXx
How many moles of CO are produced when 1.2 moles C reacts? Equation: 5C(s)+2SO2(g)→CS2(l)+4CO(g)

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
How many moles of CO are produced when 1.2 moles C reacts? Equation: 5C(s)+2SO2(g)→CS2(l)+4CO(g)...
Questions

History, 01.02.2022 14:00



Mathematics, 01.02.2022 14:00



English, 01.02.2022 14:00

Mathematics, 01.02.2022 14:00

Mathematics, 01.02.2022 14:00


Biology, 01.02.2022 14:00



Mathematics, 01.02.2022 14:00




History, 01.02.2022 14:00


Biology, 01.02.2022 14:00