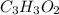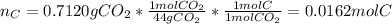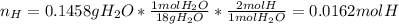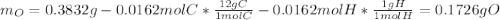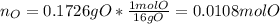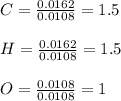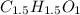Chemistry, 15.07.2020 03:01 shoreelinee1337
A 0.3832-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxygen to yield 0.7120 g of CO2 and 0.1458 g of H2O. What is the empirical formula of the compound

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
A 0.3832-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in oxy...
Questions





Chemistry, 08.10.2020 01:01






Mathematics, 08.10.2020 01:01



Mathematics, 08.10.2020 01:01


Health, 08.10.2020 01:01

Mathematics, 08.10.2020 01:01


Mathematics, 08.10.2020 01:01

English, 08.10.2020 01:01