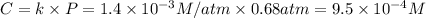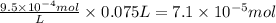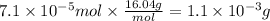Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
At 25.0°C the Henry's Law constant for methane CH4 gas in water is ×1.410−3/Matm.
Calculate the mas...
Questions

Mathematics, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00



Mathematics, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00


History, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00



Mathematics, 15.01.2021 19:00

Physics, 15.01.2021 19:00

Spanish, 15.01.2021 19:00

Mathematics, 15.01.2021 19:00


History, 15.01.2021 19:00