
Chemistry, 15.07.2020 05:01 davidb1113
What molar ratio of sodium acetate to acetic acid should be used to prepare a buffer with pH = 4.5? Ka acetic acid = 1.8 x 10-5.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
What molar ratio of sodium acetate to acetic acid should be used to prepare a buffer with pH = 4.5?...
Questions



Computers and Technology, 28.01.2021 21:40

Mathematics, 28.01.2021 21:40



English, 28.01.2021 21:40

Law, 28.01.2021 21:40

History, 28.01.2021 21:40

Mathematics, 28.01.2021 21:40

Chemistry, 28.01.2021 21:40



Mathematics, 28.01.2021 21:40

Mathematics, 28.01.2021 21:40

History, 28.01.2021 21:40

Mathematics, 28.01.2021 21:40


Mathematics, 28.01.2021 21:40

Biology, 28.01.2021 21:40

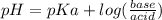 . Since we were given Ka and pH, we can find the ratio.
. Since we were given Ka and pH, we can find the ratio.

