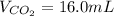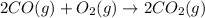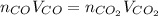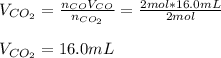Chemistry, 15.07.2020 09:01 tiaragirl923
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are produced from 16.0 mL of CO?
2 CO(g) + O2(g)
2 CO2(g)
Express your answer with the appropriate units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3
You know the right answer?
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are...
Questions

Mathematics, 02.10.2021 14:00

Business, 02.10.2021 14:00









Mathematics, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00


Mathematics, 02.10.2021 14:00


Business, 02.10.2021 14:00

Biology, 02.10.2021 14:00



Biology, 02.10.2021 14:00