
Chemistry, 15.07.2020 18:01 giselabarajas24
Sulfamic acid, HSO3NH2 (molar mass = 97.1 g/mol), is a strong monoprotic acid that can be used to standardize a strong base: A 0.179-g sample of HSO3NH2 required 19.4 mL of an aqueous solution of KOH for a complete reaction. What is the molarity of the KOH solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Sulfamic acid, HSO3NH2 (molar mass = 97.1 g/mol), is a strong monoprotic acid that can be used to st...
Questions

Mathematics, 03.09.2021 01:00

Mathematics, 03.09.2021 01:00

Chemistry, 03.09.2021 01:00

History, 03.09.2021 01:00

Mathematics, 03.09.2021 01:00



Computers and Technology, 03.09.2021 01:00



Mathematics, 03.09.2021 01:00


Mathematics, 03.09.2021 01:00

Mathematics, 03.09.2021 01:00


Mathematics, 03.09.2021 01:00

English, 03.09.2021 01:00



Mathematics, 03.09.2021 01:00


 = volume of solution in ml
= volume of solution in ml =
= 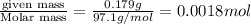

 moles of KOH
moles of KOH 



