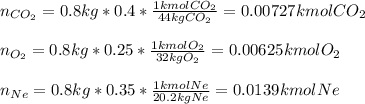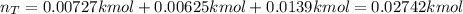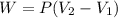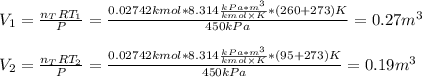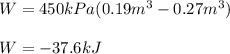A closed, frictionless piston-cylinder contains a gas mixture with the following composition on a mass basis: 40% carbon dioxide, 25% oxygen, 35% neon The cylinder contains 0.8 kg of the mixture at 260 oC and 450 kPa. Determine the magnitude and direction of work when the mixture undergoes an isobaric process to 95 oC.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
A closed, frictionless piston-cylinder contains a gas mixture with the following composition on a ma...
Questions

History, 08.10.2019 22:30

English, 08.10.2019 22:30




Social Studies, 08.10.2019 22:30


Mathematics, 08.10.2019 22:30

English, 08.10.2019 22:30



English, 08.10.2019 22:30




Social Studies, 08.10.2019 22:30