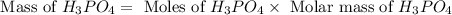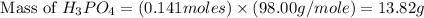Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
What mass of phosphoric acid (H3PO4, 98.00 g/mol) is produced from the reaction of 10.00 g of P4O10...
Questions





Mathematics, 19.10.2020 22:01


Mathematics, 19.10.2020 22:01

Chemistry, 19.10.2020 22:01



Computers and Technology, 19.10.2020 22:01





Mathematics, 19.10.2020 22:01




Health, 19.10.2020 22:01

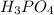 produced is, 13.82 grams.
produced is, 13.82 grams.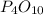 = 10.00 g
= 10.00 g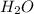 = 12.00 g
= 12.00 g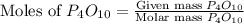
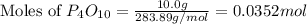
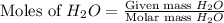
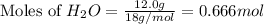
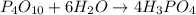
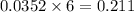 moles of
moles of 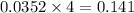 mole of
mole of