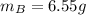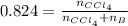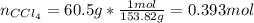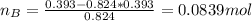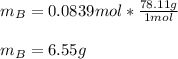Chemistry, 16.07.2020 01:01 jennamcasey94
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction of carbon tetrachloride is 0.824 in the solution obtained from 60.5 g , calculate the mass of benzene used. Mass

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1


Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

You know the right answer?
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction o...
Questions













Spanish, 12.07.2019 06:20

Mathematics, 12.07.2019 06:20


Mathematics, 12.07.2019 06:20


Social Studies, 12.07.2019 06:20