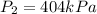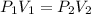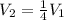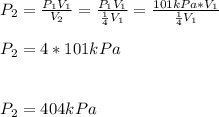Chemistry, 16.07.2020 01:01 tddreviews
Concerning Boyle's Law, if you had a gas at a pressure of 101 kPa and decreased the volume of the container holding the gas to one quarter from where it started, what would be the new pressure of the gas

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
Concerning Boyle's Law, if you had a gas at a pressure of 101 kPa and decreased the volume of the co...
Questions



Mathematics, 06.11.2020 19:20


Mathematics, 06.11.2020 19:20



History, 06.11.2020 19:20

Mathematics, 06.11.2020 19:20


Mathematics, 06.11.2020 19:30


Mathematics, 06.11.2020 19:30


Social Studies, 06.11.2020 19:30



Advanced Placement (AP), 06.11.2020 19:30