
Chemistry, 13.10.2019 23:50 ANONYMUSNESS8670
The sodium atom loses an electron to form a sodium ion (na+). which statement is correct with respect to its atomic radius?
the sodium ion has a larger radius than the atom.
the sodium ion has a smaller radius than the atom.
the sodium ion and the sodium atom radii are the same size.
the sodium ion has twice the radius of the sodium atom.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
The sodium atom loses an electron to form a sodium ion (na+). which statement is correct with respec...
Questions

Mathematics, 02.09.2020 19:01


English, 02.09.2020 19:01

Computers and Technology, 02.09.2020 19:01

Mathematics, 02.09.2020 19:01


History, 02.09.2020 19:01

English, 02.09.2020 19:01

Biology, 02.09.2020 19:01

Mathematics, 02.09.2020 19:01

Mathematics, 02.09.2020 19:01


Mathematics, 02.09.2020 19:01







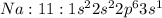
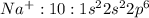
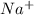 has 10 electrons and 11 protons. Now 11 protons present in the nucleus can easily influence 10 electrons towards itself, the effective nuclear charge increases, the valence electrons are more tightly held by the nucleus and thus the size decreases.
has 10 electrons and 11 protons. Now 11 protons present in the nucleus can easily influence 10 electrons towards itself, the effective nuclear charge increases, the valence electrons are more tightly held by the nucleus and thus the size decreases.

