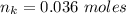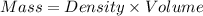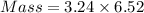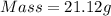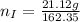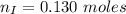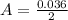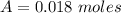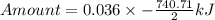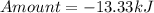Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
How much heat is liberated at constant pressure when 1.41 g of potassium metal reacts with 6.52 mL o...
Questions

Engineering, 16.07.2020 20:01



Mathematics, 16.07.2020 20:01










English, 16.07.2020 20:01



Mathematics, 16.07.2020 20:01


Advanced Placement (AP), 16.07.2020 20:01

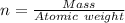
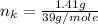 ---where 39 is the atomic weight of potassium
---where 39 is the atomic weight of potassium