Chemistry, 16.07.2020 05:01 AleciaCassidy
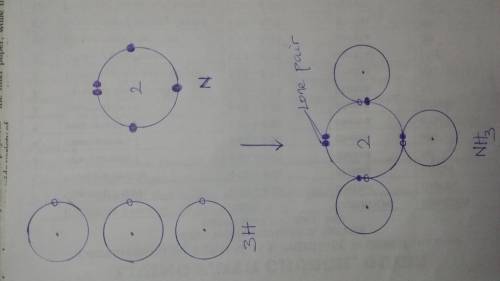
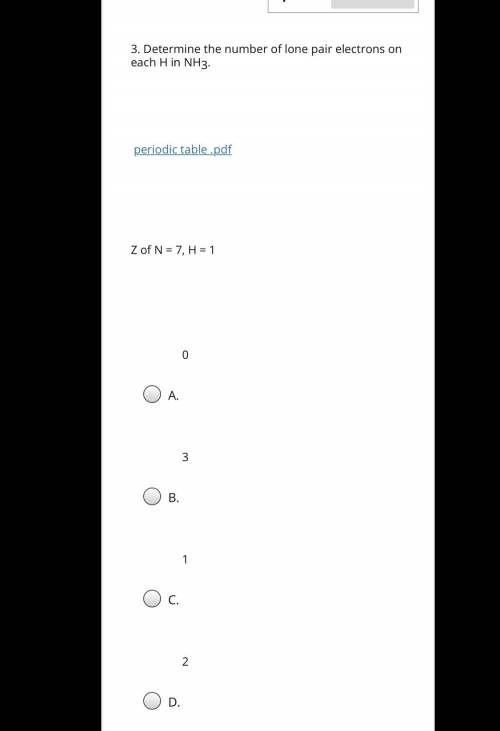
Determine the number of lone pair electrons on each H in NH3. Z of N= 7, H=1 A. 0 B. 3 C. 1 D. 2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
Determine the number of lone pair electrons on each H in NH3. Z of N= 7, H=1 A. 0 B. 3 C. 1 D. 2
Questions

Mathematics, 11.06.2021 17:50





Mathematics, 11.06.2021 17:50



English, 11.06.2021 17:50

Physics, 11.06.2021 17:50


English, 11.06.2021 17:50


Biology, 11.06.2021 17:50

English, 11.06.2021 17:50


Mathematics, 11.06.2021 18:00

Mathematics, 11.06.2021 18:00