
Chemistry, 16.07.2020 17:01 payshencec21
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Calculate the equilibrium concentrations of reactants and product when 0.234 moles of H2 and 0.234 moles of I2 are introduced into a 1.00 L vessel at 698 K.
[H2] = M
[I2] = M
[HI] = M

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Questions

Mathematics, 18.09.2020 06:01

English, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

English, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Chemistry, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

English, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

English, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

German, 18.09.2020 06:01

Physics, 18.09.2020 06:01

Mathematics, 18.09.2020 06:01

![[I_2]=[H_2]=0.369M](/tpl/images/0707/8736/ed0a2.png)
![[HI]=0.0495M](/tpl/images/0707/8736/4fca5.png)
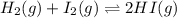
![Kc=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0707/8736/bf8a4.png)
 (considering the ICE procedure) is written as:
(considering the ICE procedure) is written as:![55.6=\frac{(2x)^2}{([I_2]_0-x)([H_2]_0-x)}](/tpl/images/0707/8736/e5efd.png)
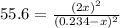
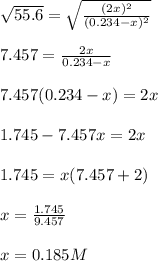
![[I_2]=[H_2]=2*0.185M=0.369M](/tpl/images/0707/8736/ec590.png)
![[HI]=0.234-0.185=0.0495M](/tpl/images/0707/8736/318f7.png)


