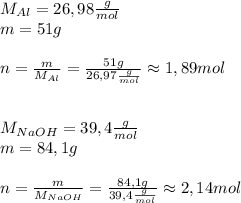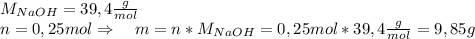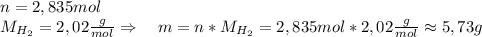Asolid piece of aluminum (51.0 g) was added to a solution of sodium hydroxide (84.1 g) in water, a balanced equation for this reaction is shown below:
2 naoh(aq)+ 2 al(s)+ 2 h2o → 2 naalo2(aq)+ 3 h2(g)
(a) which reagent is completely consumed by the reaction?
(b) after the reaction is completed, what is the mass of the reagent that remains? (c) what mass of hydrogen gas is produced?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
Asolid piece of aluminum (51.0 g) was added to a solution of sodium hydroxide (84.1 g) in water, a b...
Questions

History, 13.11.2020 16:00


Social Studies, 13.11.2020 16:00

Biology, 13.11.2020 16:00

History, 13.11.2020 16:00


Physics, 13.11.2020 16:00


Social Studies, 13.11.2020 16:00


Mathematics, 13.11.2020 16:00


Mathematics, 13.11.2020 16:00



Mathematics, 13.11.2020 16:00


Mathematics, 13.11.2020 16:00