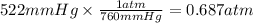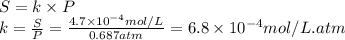Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
The solubility of nitrogen gas at 25°C and a nitrogen pressure of 522 mmHg is 4.7 × 10 –4 mol/L. Wha...
Questions

History, 14.07.2019 12:20

History, 14.07.2019 12:20





Biology, 14.07.2019 12:20



Mathematics, 14.07.2019 12:20

History, 14.07.2019 12:20

Biology, 14.07.2019 12:20

History, 14.07.2019 12:20



Social Studies, 14.07.2019 12:20

English, 14.07.2019 12:20

Spanish, 14.07.2019 12:20