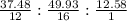A substance used as a cleaner and a fuel is 37.48% C, 49.93% O and 12.58% H by mass. A 0.2804-g sample of the substance occupies a volume of 250.0 mL when it is vaporized at 75o C and 1.00 atm of pressure.
R = 0.0821 L atm/ mol K
a) This compound can be made by combining gaseous carbon monoxide with hydrogen gas (with this compound as the only product). What is the maximum mass of this compound that can be prepared if 8.0 kg of hydrogen gas react with 59.0 kg of carbon monoxide gas?
b) If 59.6 kg of the product is actually produced, given the reaction described in (a), what is the percent yield?
c) This compound (the substance you identified in part a) is a potential replacement for gasoline. The products of the complete combustion of this fuel are the same as those for the complete combustion of a hydrocarbon (CO2 and H2O). Calculate the volume of CO2 produced at 27o C and 766 mmHg when 1.00 gallon of this fuel is completely combusted. The density of the fuel is 0.7914 g/mL. 1 gallon = 3.785 liters
d) A claim was made that this fuel is better for the environment because it produces less CO2 per gallon than gasoline, which can be represented by the formula C8H18 (octane). Is this claim true? Octane has a density of 0.6986 g/mL

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
A substance used as a cleaner and a fuel is 37.48% C, 49.93% O and 12.58% H by mass. A 0.2804-g samp...
Questions


Health, 04.07.2019 11:10

Mathematics, 04.07.2019 11:10


Chemistry, 04.07.2019 11:10


Biology, 04.07.2019 11:10

Business, 04.07.2019 11:10

Mathematics, 04.07.2019 11:10


Mathematics, 04.07.2019 11:10

English, 04.07.2019 11:10

Mathematics, 04.07.2019 11:20

Mathematics, 04.07.2019 11:20




Mathematics, 04.07.2019 11:20

English, 04.07.2019 11:20

Chemistry, 04.07.2019 11:20