Chemistry, 20.07.2020 01:01 cheesecake1919
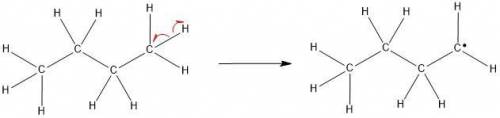
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG (Liquefied Petroleum Gas) cylinders (a common source of cooking gas). It has two arrangements of carbon atoms: a straight chain and a branched chain. Using this information, draw the structure of the tertiary butyl radical that will form upon removal of a hydrogen atom.
Requried:
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The molecular formula of butane is C4H10. It is obtained from petroleum and is used commonly in LPG...
Questions






Social Studies, 28.07.2019 09:00




Geography, 28.07.2019 09:00

English, 28.07.2019 09:00









Mathematics, 28.07.2019 09:00