Chemistry, 19.07.2020 14:01 umezinwachukwuebuka1
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as the solvent and the other has carbon tetrachloride as the solvent. Determine that molal concentration, m (or b), and boiling point, Tb.
Given
Ethanol
normal Boiling point: 78.4
Kb: 1.22
CCL4
normal boiling point: 76.8
Kb: 5.03

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as...
Questions



Mathematics, 29.01.2021 01:30



Arts, 29.01.2021 01:30



English, 29.01.2021 01:30

Chemistry, 29.01.2021 01:30


Mathematics, 29.01.2021 01:30


Biology, 29.01.2021 01:30

Mathematics, 29.01.2021 01:30




Chemistry, 29.01.2021 01:30

Mathematics, 29.01.2021 01:30

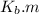
 is the molal boiling point elevation constant;
is the molal boiling point elevation constant;