Chemistry, 19.07.2020 17:01 Aurionna101
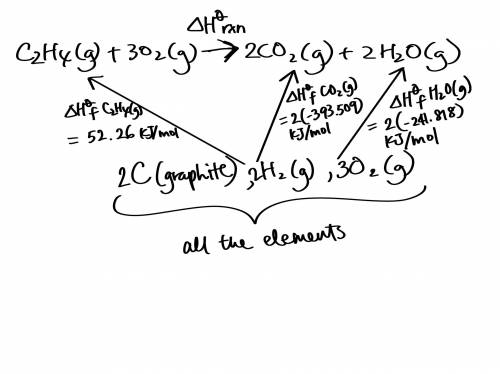
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for the following reaction. (The ΔHf of C2H4 is 52.26 kJ/mol, CO2 is -393.509 kJ/mol, and H2O is -241.818 kJ.)
C2H4 (g) + 3O2(g) 2CO2 (g) + 2H2O(g)
ΔHrxn = (-345.64 kJ, -583.07 kJ, or -1,322.91 kJ).
The reaction is: (Endothermic or Exothermic).

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for t...
Questions



Physics, 12.03.2020 05:46
















Mathematics, 12.03.2020 05:47