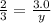Chemistry, 20.07.2020 07:01 memester74
A 3.0 mole sample of KCIO3 was decomposed according to the equation:
2KCIO3(s) ===> 2KCI(s) + 3O2(g)
How many moles of oxygen (O2) will be formed?
o 2.5 moles
4.0 moles
3.0 moles
2.0 moles
4.5 moles

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
A 3.0 mole sample of KCIO3 was decomposed according to the equation:
2KCIO3(s) ===> 2KCI(s) + 3O...
Questions




Health, 26.08.2019 05:30

Mathematics, 26.08.2019 05:30

Spanish, 26.08.2019 05:30

English, 26.08.2019 05:30




Chemistry, 26.08.2019 05:30


Mathematics, 26.08.2019 05:30


History, 26.08.2019 05:30

History, 26.08.2019 05:30




Mathematics, 26.08.2019 05:50