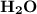Indicate the types of forces that are involved between the solute and solvent when forming a homogeneous solution between CH3CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH3.
a. dispersion forces
b. dipole-dipole forces
c. hydrogen bonding
d. ion-dipole forces
2. Indicate the types of forces that are involved between the solute and solvent when forming a homogeneous solution between LiNO3 and H2O.
a. dispersion forces
b. dipole-dipole forces
c. hydrogen bonding
d. ion-dipole forces

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Indicate the types of forces that are involved between the solute and solvent when forming a homogen...
Questions

Mathematics, 21.05.2020 00:12




History, 21.05.2020 00:12

Mathematics, 21.05.2020 00:12




Computers and Technology, 21.05.2020 00:12




Mathematics, 21.05.2020 00:12

History, 21.05.2020 00:12




Computers and Technology, 21.05.2020 00:12

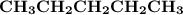 and
and 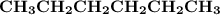
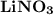 and
and