
Chemistry, 22.07.2020 06:01 faithtunison
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a
75.0 L tank with 3.8 mol of sulfur dioxide gas and 7.0 mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of sulfur trioxide
gas to be 1.5 mol
Calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2
significant digits.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions


Computers and Technology, 04.01.2021 14:40

Social Studies, 04.01.2021 14:40

Geography, 04.01.2021 14:40

Business, 04.01.2021 14:40

Biology, 04.01.2021 14:40


Mathematics, 04.01.2021 14:40

English, 04.01.2021 14:40

Mathematics, 04.01.2021 14:40


Social Studies, 04.01.2021 14:40




Mathematics, 04.01.2021 14:40




Physics, 04.01.2021 14:40

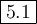
![K_{\text{eq}} = \dfrac{\text{[SO$_{3}$]}^{2}}{\text{[SO}_{2}]^{2}\text{[O$_{2}$]}} = \dfrac{0.0200^{2}}{0.0307^{2}\times0.0833} =\mathbf{5.1}\\\\\text{The value of the equilibrium constant is $\large \boxed{\mathbf{5.1}}$}](/tpl/images/0711/0231/1d368.png)


