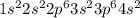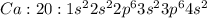Chemistry, 22.07.2020 20:01 yselahernandez02
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. Use the rules for determining electron configurations to write the electron configuration for Ca. Express your answer in complete form in order of orbital filling. For example, 1s22s2 should be entered as 1s^22s^2.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons...
Questions

English, 29.10.2020 18:00

English, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00

English, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00

Mathematics, 29.10.2020 18:00



Mathematics, 29.10.2020 18:00


Mathematics, 29.10.2020 18:00


History, 29.10.2020 18:00



Mathematics, 29.10.2020 18:00

English, 29.10.2020 18:00


Mathematics, 29.10.2020 18:00