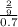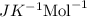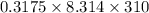Chemistry, 22.07.2020 23:01 MidnightAIY179
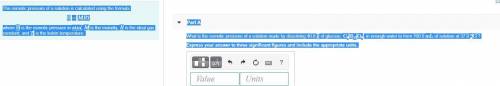
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pressure in atm, M is the molarity, R is the ideal gas constant, and T is the kelvin temperature. Part A What is the osmotic pressure of a solution made by dissolving 40.0 g of glucose, C6H12O6, in enough water to form 700.0 mL of solution at 37.0 ∘C ? Express your answer to three significant figures and include the appropriate units. nothing nothing

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pres...
Questions

Mathematics, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00

History, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00


English, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00

History, 02.12.2021 01:00

Computers and Technology, 02.12.2021 01:00


Computers and Technology, 02.12.2021 01:00


Health, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00




Business, 02.12.2021 01:00

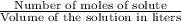
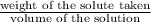
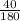
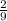 moles
moles