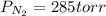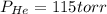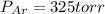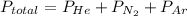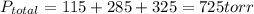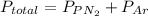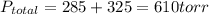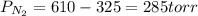A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pressures of 115, 285, and 325 torr, respectively. If all the He is removed from the mixture and the temperature does not change, what will be the partial pressure, in torr, of the N2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pre...
Questions

Arts, 25.04.2020 01:09




Mathematics, 25.04.2020 01:09






Mathematics, 25.04.2020 01:09

Mathematics, 25.04.2020 01:09


Chemistry, 25.04.2020 01:09



Mathematics, 25.04.2020 01:09


English, 25.04.2020 01:09

Mathematics, 25.04.2020 01:09