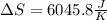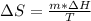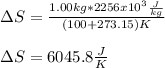Chemistry, 23.07.2020 01:01 janellesteele7498
Calculate the change in entropy when 1.00 kgkg of water at 100∘C∘C is vaporized and converted to steam at 100∘C∘C. Assume that the heat of vaporization of water is 2256×103J/kg2256×103J/kg. Express your answer in joules per kelvin.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Calculate the change in entropy when 1.00 kgkg of water at 100∘C∘C is vaporized and converted to ste...
Questions


Mathematics, 20.11.2020 23:30

History, 20.11.2020 23:30

Arts, 20.11.2020 23:30



English, 20.11.2020 23:30


Chemistry, 20.11.2020 23:30

Mathematics, 20.11.2020 23:30




Chemistry, 20.11.2020 23:30


Mathematics, 20.11.2020 23:30


English, 20.11.2020 23:30


Mathematics, 20.11.2020 23:30