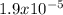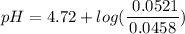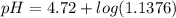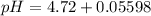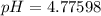Chemistry, 23.07.2020 19:01 moneyyfletcher
A 25.0-mL sample of 0.150 M hydrazoic acid, HN3, is titrated with a 0.150 M NaOH solution. What is the pH after 13.3 mL of base is added? The Ka of hydrazoic acid = 1.9 x 10-5.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
A 25.0-mL sample of 0.150 M hydrazoic acid, HN3, is titrated with a 0.150 M NaOH solution. What is t...
Questions



Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00

Health, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00

Business, 18.03.2021 19:00

Social Studies, 18.03.2021 19:00

Business, 18.03.2021 19:00

History, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00

Computers and Technology, 18.03.2021 19:00


Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00


Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00


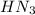 =
= 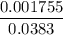 = 0.0458 M
= 0.0458 M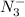 =
= 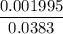 = 0.0521 M
= 0.0521 M